Chapter Nineteen: Metabolic Acidosis, part 3
Edited by Nayan Arora
References
Chapter 19, Part 3 August 30, 2023
Joel and Roger mentioned the most common cause seems to be Sjögren’s syndrome for an acquired distal RTA. We mentioned this in an earlier episode and referenced this example of an absence of the H+ ATPase, presumably from autoantibodies to this transporter. Here’s a case report: Absence of H(+)-ATPase in cortical collecting tubules of a patient with Sjogren's syndrome and distal renal tubular acidosis
Joel mentioned this paper in the New England Journal of Medicine in which there were patients who had hyperkalemia with a distal RTA: Hyperkalemic Distal Renal Tubular Acidosis Associated with Obstructive Uropathy | NEJM in this setting, some patients
Anna mentioned this article on “ampho-terrible:” It’s the holes!!! Yano T, Itoh Y, Kawamura E, Maeda A, Egashira N, Nishida M, Kurose H, Oishi R. Amphotericin B-induced renal tubular cell injury is mediated by Na+ Influx through ion-permeable pores and subsequent activation of mitogen-activated protein kinases and elevation of intracellular Ca2+ concentration. Antimicrob Agents Chemother. 2009 Apr;53(4):1420-6
Josh mentioned this study on furosemide’s effect on the TAL: Furosemide-induced urinary acidification is caused by pronounced H+ secretion in the thick ascending limb
Melanie mentioned treatment of patients with cystinosis Expert guidance on the multidisciplinary management of cystinosis in adolescent and adult patients | Clinical Kidney Journal | Oxford Academic
Amy shared her observations regarding base supplements including Prevention of recurrent calcium stone formation with potassium citrate therapy in patients with distal renal tubular acidosis - PubMed and Dosage of potassium citrate in the correction of urinary abnormalities in pediatric distal renal tubular acidosis patients - PubMed
Roger mentioned that he has had good luck with Moonstone Nutrition drinks alkali citrates for kidney health
We referred to David Goldfarb’s teaching on kidney stones in patients with acidification defects: A Woman with Recurrent Calcium Phosphate Kidney Stones (we also referenced this in an earlier episode but this one is a fan favorite).
Joel mentioned the concern of bone loss in distal RTA: Incomplete renal tubular acidosis in 'primary' osteoporosis and Abnormal distal renal tubular acidification in patients with low bone mass: prevalence and impact of alkali treatment
Lety mentioned concerns of encrustation of stents in stone forming individuals Potassium Citrate as a Preventive Treatment for Double-J Stent Encrustation: A Randomized Clinical Trial
Joel schooled us in toluene and the presentation which appears to be an RTA- https://journals.lww.com/JASN/Abstract/1991/02000/Glue_sniffing_and_distal_renal_tubular_acidosis_.3.aspx
Melanie mentioned this work by Alan Yu’s lab on a mechanism of hypercalciuria Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease
Furosemide/Fludrocortisone Test and Clinical Parameters to Diagnose Incomplete Distal Renal Tubular Acidosis in Kidney Stone Formers and an accompanying editorial by Goldfarb Refining Diagnostic Approaches in Nephrolithiasis: Incomplete Distal Renal Tubular Acidosis
Here’s a nice piece on ifosfamide and phosphate from Josh New clues for nephrotoxicity induced by ifosfamide: preferential renal uptake via the human organic cation transporter 2
Here’s this crazy piece on excessive bicarbonate - Gas production after reaction of sodium bicarbonate and hydrochloric acid
Josh points out that the pH can be important for inotropy: An effect of pH upon epinephrine inotropic receptors in the turtle heart
Mel’s favorite from Halperin because of the pun: Renal tubular acidosis (RTA): recognize the ammonium defect and pHorget the urine pH
Amy’s VOG on RTA and Osteoporosis
KI Review on acidosis and bone health: Effects of acid on bone
Guideline on congenital RTA: Distal renal tubular acidosis: ERKNet/ESPN clinical practice points
AJKD article on acidosis and bone health: Serum Bicarbonate and Bone Mineral Density in US Adults
Citrate reversing CsA induced acidosis effects: Citrate reverses cyclosporin A-induced metabolic acidosis and bone resorption in rats
Outline: Chapter 19 Metabolic Acidosis part 3
Renal Tubular Acidosis
Acidosis from diminished net tubular acid secretion
Three types
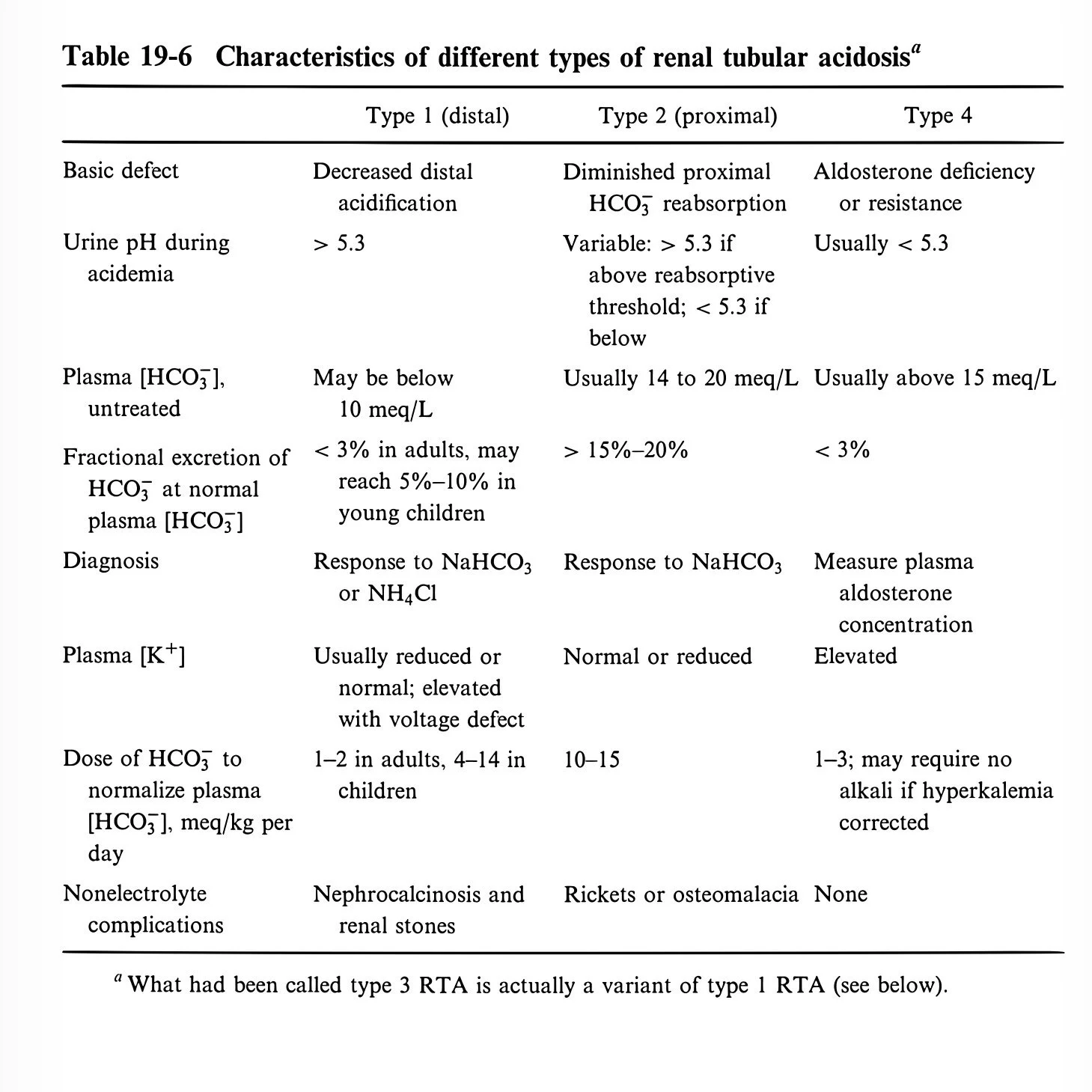
Type 1 (Distal)
Type 2 (Proximal)
Type 4 (…)
The acidosis of renal failure could be added to this group
But NH4+ per nephron is normal
This is a problem of too few nephrons, not tubular acidosis
Nephrons able to maximally acidify the urine
Type 1 Distal RTA
Decrease in net H secretion in the collecting duct
Minimal urine pH rises from 4.5 to 5.3
HCO3 can fall below 10
Three mechanisms
Defect in H-ATPase found in cortex and medulla
Sjögren syndrome
Can be genetic chloride bicarbonate exchanger
This pumps bicarbonate out basolateral membrane after it is generated in the splitting of water to form H
Defect in cortical Na reabsorption
Voltage-dependent defect
Concurrent K secretion defect
Found in urinary obstruction and sickle cell
Volume deficiency can decrease Na delivery to distal nephron
Decreased amount of Na reabsorption can cause a reversible type 1 RTA of this type
Increased membrane permeability
Amphotericin
pH of 5.0 is 250× plasma
Table 19-7
Fractional excretion of bicarbonate in distal RTA
Normally negligible since bicarbonate can’t exist with pH down around 5
In distal RTA it may be as high as 6.5; FEHCO3 is 3%
If pH goes up over 7 this can rise to 5–10%
Usually in infants
As they age their urine pH falls a bit
This is called type 3
Plasma K
H-ATPase defects have low K
Patients also have downregulation of H-K-ATPase
Downregulation of NaCl reabsorption in proximal tubule
Decreased filtered bicarbonate means less bicarbonate to absorb with Na, hence more Na excretion from proximal tubule
This increases distal sodium delivery and increases aldosterone
Voltage defect also has decreased renal K clearance → hyperkalemia
Differentiate from type 4 RTA by looking at urine pH
Lower in type 4
Higher in voltage-dependent distal RTA
Nephrocalcinosis
Hypercalciuria, hyperphosphatemia, nephrolithiasis, and nephrocalcinosis are frequent
Comes from bones buffering the acidosis
Kidney decreases reabsorption of these so they are lost in urine
Two other factors
Low urinary citrate
Hypokalemia drives this
Acidosis drives this
High urine pH (CaPhos stones)
All corrected by correcting the metabolic acidosis
Incomplete Type 1
Defective urinary acidification but not acidemic
Increased proximal NH3 production lowers urinary H
Low urinary citrate
Can progress to complete type 1
Etiology of Type 1
Sjögren syndrome, rheumatoid arthritis
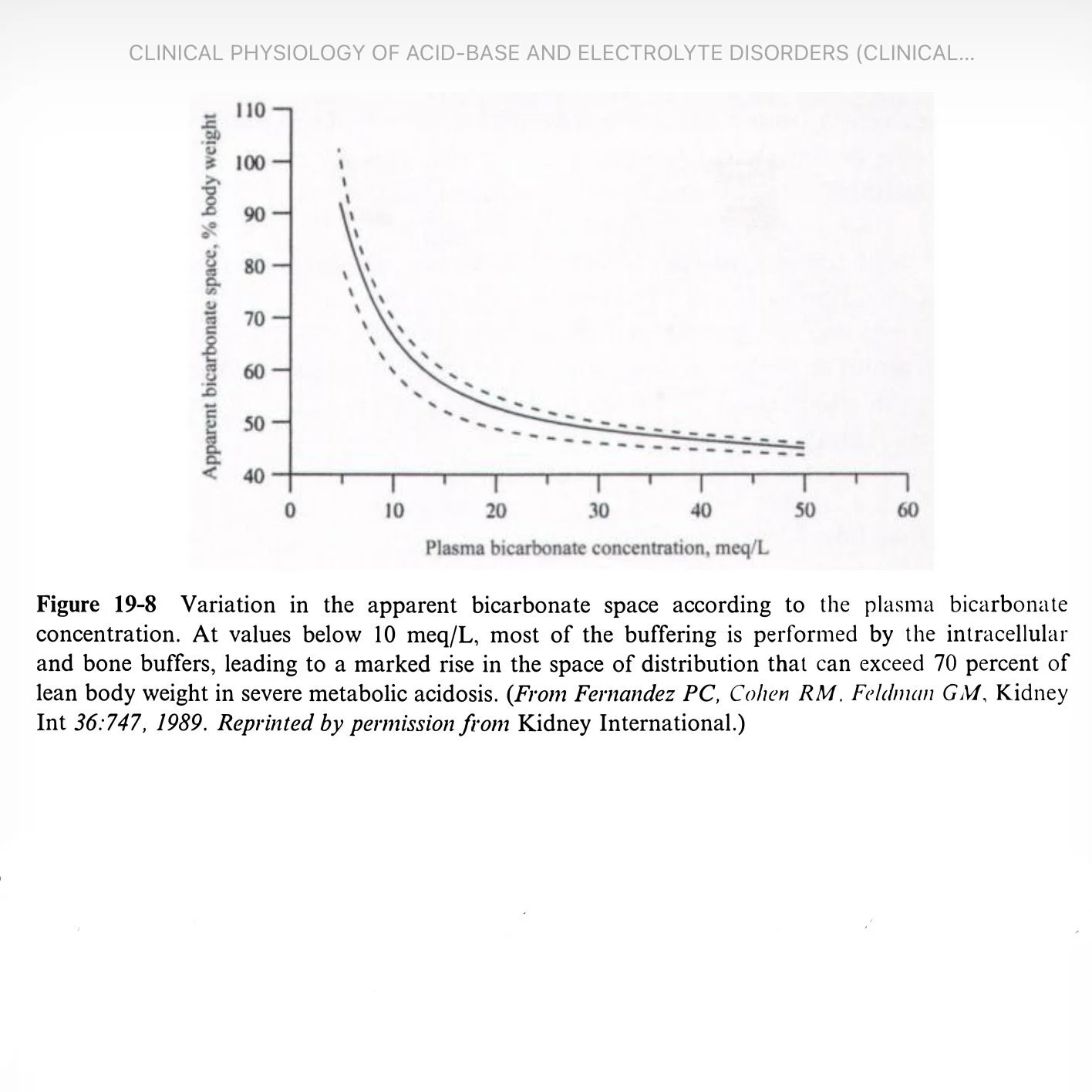
19-8
Clinical manifestations
Stones
Hypokalemia
Growth defects
Diagnosis
NAGMA and elevated urine pH
5.3 in adults
5.6 in children
Differentiate Type 1 vs Type 2
Give bicarbonate drip
1 mEq/kg/hr
Urine pH remains high with Type 1
Does not go up as it does with proximal Type 2
Incomplete distal RTA
Give acid load
0.1 mmol/kg
Urine pH remains >5.3 in classic
Falls in normal patients (usually below 5)
Treatment
Treat metabolic acidosis
Minimize potassium loss
Reduce bone catabolism
Prevent stones
Alkali requirement
Adults: 1–2 mEq/kg/day
Children: 4–14 mEq/kg/day
Alkali
Sodium bicarbonate
Sodium citrate
Potassium citrate if hypokalemia persists despite correcting acidosis
Or for calcium stone disease
Treat hypokalemia
Type 2 Proximal RTA
Decreased HCO3 reabsorption
90% of bicarbonate reabsorption happens in proximal tubule
Bicarbonate wasting starts normally at 26–28 mmol/L (Tm for bicarbonate)
In RTA 2 the Tm falls to a lower level (maybe 17)
Serum bicarbonate falls to 17 and stabilizes
Type 2 RTA is self-limiting
Typically HCO3 around 14–20
Distal acidification intact
Carbonic anhydrase inhibitor can block 80% of proximal HCO3 reabsorption
Only 30% of filtered bicarbonate excreted due to distal H secretion
Total absence of proximal reabsorption results in HCO3 11–12
Clinical difference in treatment
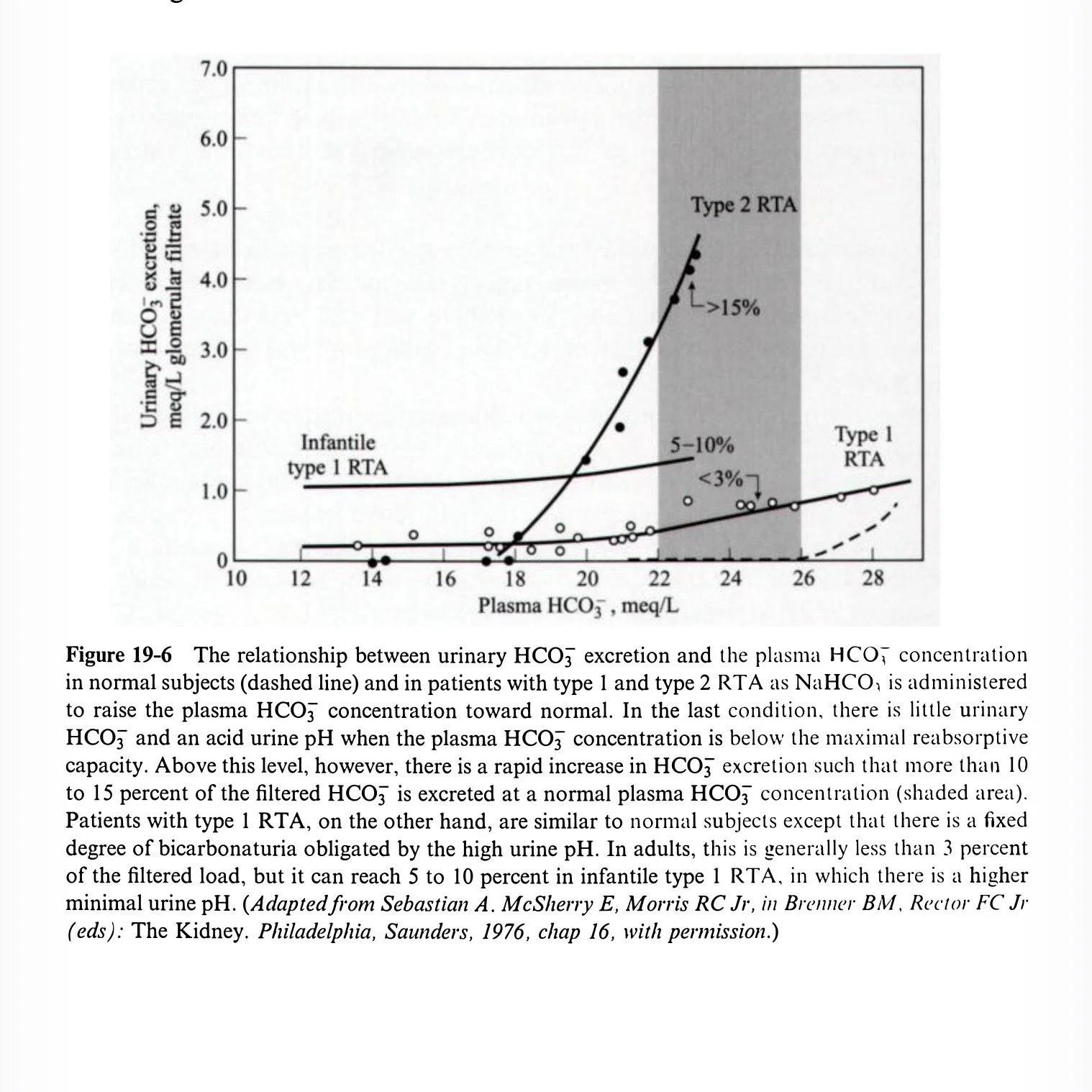
In Type 2, giving bicarbonate and raising serum HCO3 above Tm → more wasted in urine
FEHCO3 can reach 15% with normal serum HCO3
Urine pH >7.5
Below Tm, urine pH <5.3
In Type 1, curve relating HCO3 excretion to plasma HCO3 similar to normal (with increased obligatory urine HCO3 due to higher urine pH)
Defect in HCO3 reabsorption
Can be isolated
Or part of Fanconi syndrome
Pathogenesis (three steps)
Na-H exchange (apical membrane)
Na-K-ATPase (basolateral membrane)
Carbonic anhydrase
Intracellular
Luminal
Multiple myeloma most common adult cause
Ifosfamide
Can also cause phosphate wasting, NDI, and Type 1 RTA
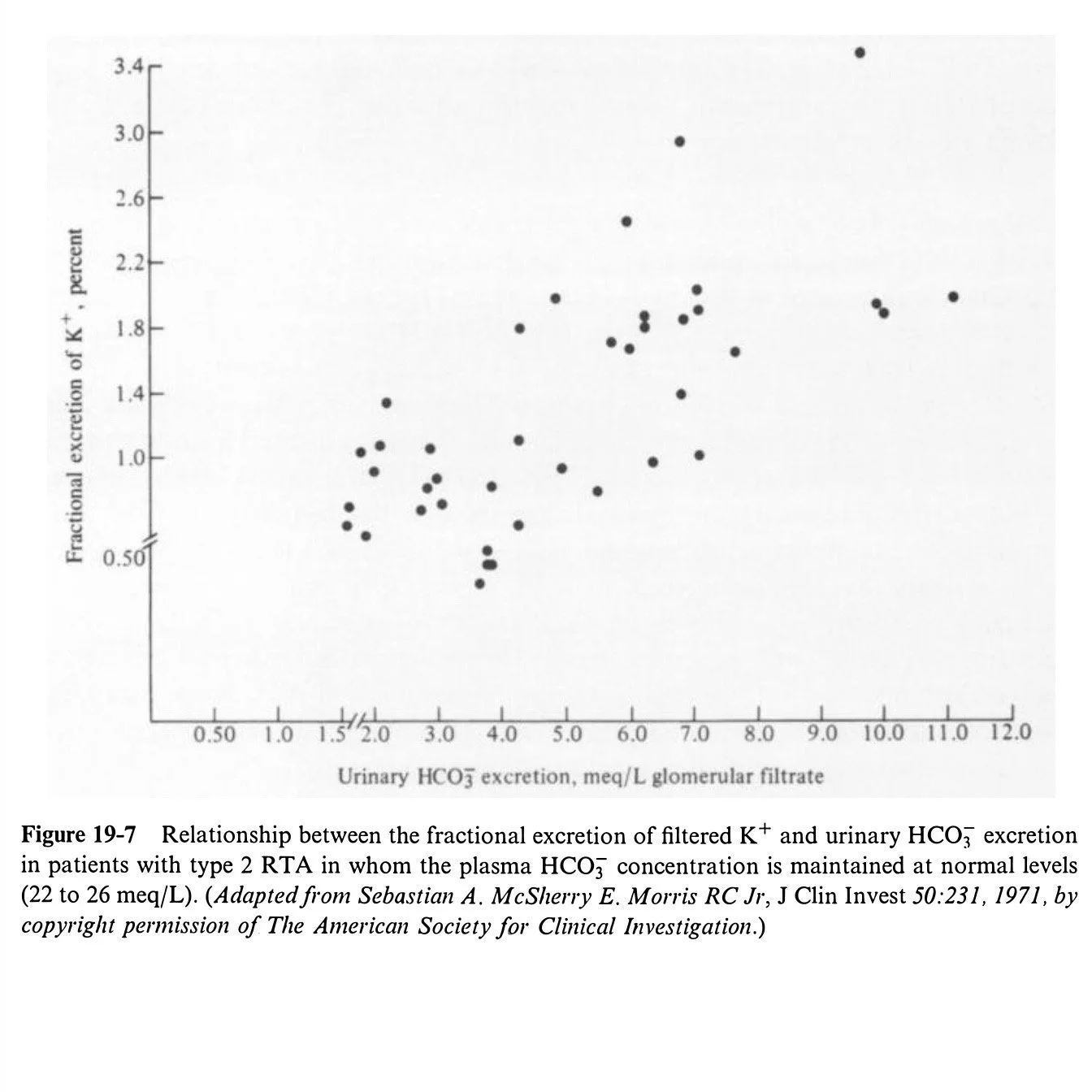
K balance
Common but variable
Mild hypokalemia at baseline due to increased Na wasting → hyperaldosteronism
Worse with bicarbonate therapy
Distal delivery of nonreabsorbable anion increases obligate cation loss
Figure 19-7
Bone disease
Rickets (children), osteomalacia/osteopenia (adults)
Up to 20%
Phosphate wasting and vitamin D deficiency may contribute
Impaired growth
No nephrocalcinosis or nephrolithiasis
Lower urine pH
Nonreabsorbable amino acids and organic anions bind calcium
Etiology
19-9
Idiopathic and cystinosis (children)
Carbonic anhydrase inhibitors
Multiple myeloma
Diagnosis
NAGMA and pH <5.3
Look for Fanconi syndrome
Raise serum HCO3 and watch urine pH rise
FEHCO3 15–20%
Treatment
Correct acidosis to allow normal growth
Difficult due to rapid urinary loss
May need 10–15 mEq/kg/day
HCO3 or citrate
More than 20 mEq HCO3 can cause stomach rupture from CO2 generation
Small dose thiazide to increase proximal Na reabsorption and HCO3 reabsorption
Idiopathic Type 2 may improve after years
Type 4 RTA
Aldosterone deficient or resistant
Normally stimulates H secretion and K secretion
Loss causes hyperkalemia and metabolic acidosis
Hyperkalemia antagonizes NH4 generation
High K may outcompete NH4 on Na-K-2Cl in TALH
Less ammonium recycling
Less NH3 available in collecting duct
Correcting hyperkalemia can correct acidosis
Metabolic acidosis generally mild
HCO3 >15
Urine pH <5.3 (generally, not always)
Mineralocorticoid can treat but causes hypertension and sodium retention
Often responds to loop diuretic
Rhabdomyolysis can cause high anion gap metabolic acidosis
Symptoms
Respiratory compensation increases 4–8 fold → dyspnea
pH <7.0–7.1
Fatal ventricular arrhythmias
Reduced cardiac contractility
Decreased response to inotropes
Neurological
Lethargy to coma
More related to CSF pH than plasma
Less neurologic symptoms than respiratory acidosis
BBB more permeable to CO2 than HCO3
Skeletal problems
Decreased growth
Kids/infants: anorexia, nausea, listlessness
Treatment
General principles
Correct with HCO3
No alkali required for lactic or ketoacidosis
Goal: pH >7.2
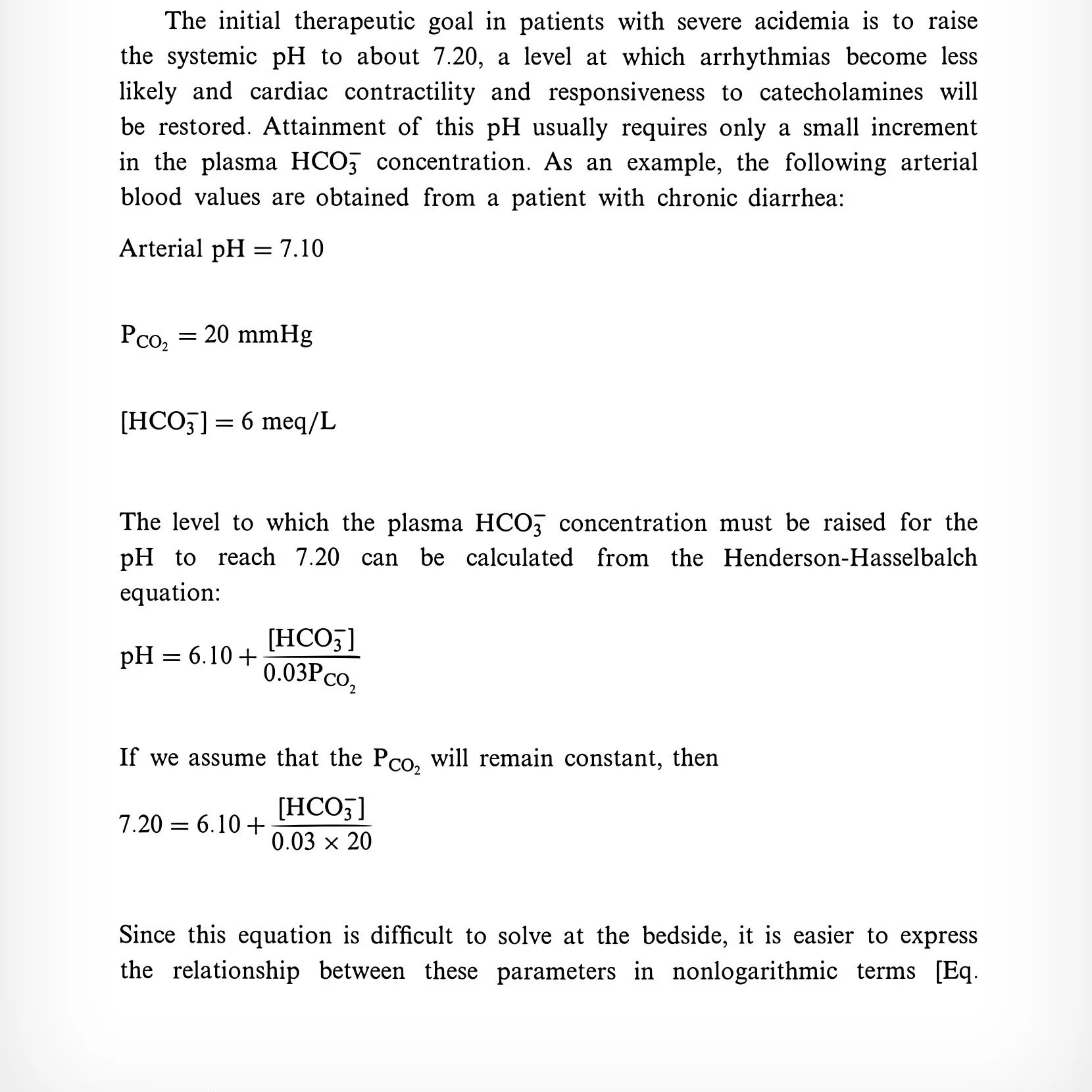
Equations on page 629 need “log”
Example: pH 7.1, pCO2 20, HCO3 6
Raise HCO3 to 8 if pCO2 stays 20
Raise to 10 if pCO2 rises
Paragraph “regardless…” highlights risks of bicarbonate
Bicarbonate deficit
Deficit = HCO3 space × HCO3 deficit per liter
HCO3 space
50% body weight (normal)
60% (mild–moderate acidosis)
70% (severe, HCO3 <8–10)
Example: 70 kg, raise HCO3 6→10 using 0.7 space = 196 mEq
Rough guideline; does not account for ongoing acid production
Early large bump in bicarbonate
Drifts down as bicarbonate moves intracellularly
Plasma potassium
K depletion can cause metabolic acidosis
Metabolic acidosis increases K
“Normal” K may mask depletion (see DKA)
Beware correcting acidosis in hypokalemia
Heart failure
Bicarbonate comes with sodium load
Comment that bicarbonate moves into cell
But Na remains extracellular
Dialysis can be used