Chapter One: Introduction to Renal Function
Please enjoy the first episode of our book club. Join us as we start our journey through The Clinical Physiology of Acid Base and Electrolyte Disorders.
Chapter outline:
Hello and welcome to chapter one: Introduction to Renal Function
Summary of kidney functions
Maintenance of extracellular environment
Hormone secretion
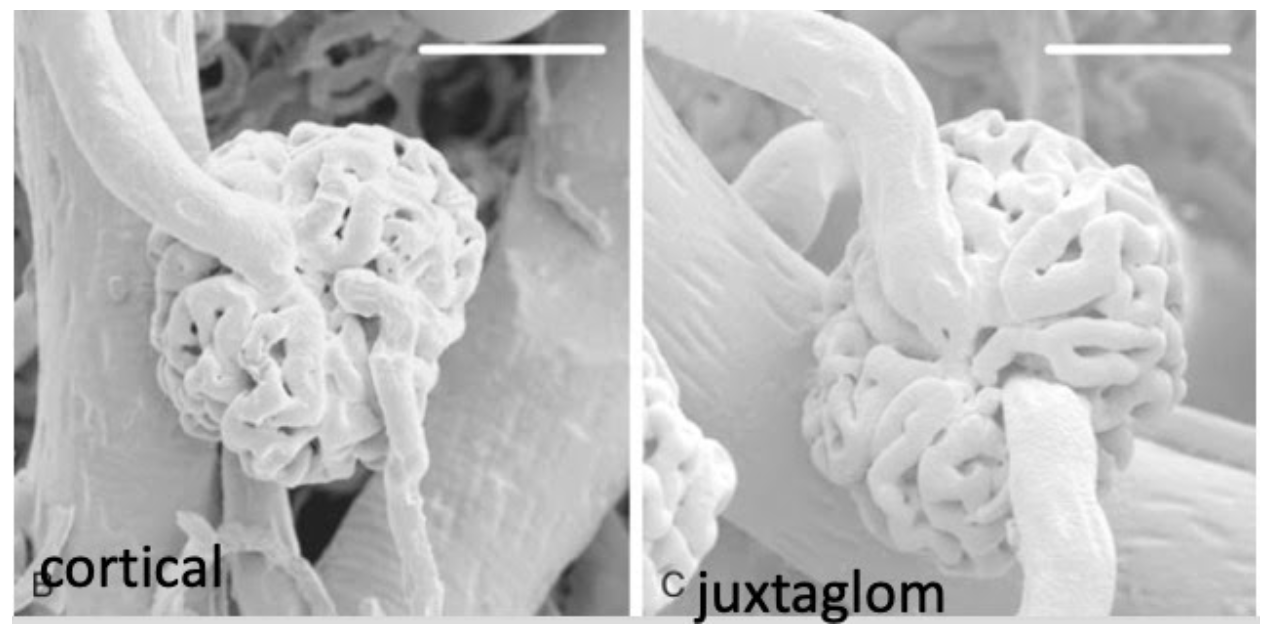
Renin
Ang2
PGE
NO
Endothelin
Bradykinin
Epo
1,25 D
Is soluble Klotho another hormone that should be added to this list?
Misc: catabolism of protein and gluconeogenesis
Is that all?
Where’s BP in that list?
Renal Morphology
Introduces the nephron
Funny. No simple definition of a nephron
Cortex and medulla
From Brenner Rector
I take it those white bars at the top mean these are at the same magnification?
Reabsorption and secretion
Proximal tubule
Loop
Long vs short loops
DCT
CCT
Duct vs Tubule
Reabsorption and Secretion
Paracellular vs across the cell
Is there such a thing as paracellular secretion?
Typo page 8 last paragraph, describing NaK2Cl as being in the CCT rather than LOH
Why do we filter so much only to reabsorption 98-99% of the water, sodium chloride and bicarb
Love table 1-1
The role of the tight junction (see detailed dive by Josh below)
Comparison of leaky TJ in PT and
Tight TJ in MCT
Then there is the Molitores section
Membrane recycling
Composition of the urine
The composition of the extracellular compartment is constant because of the variability of the content of the urine
Atomic weight and molarity
Equivalence
Osmotic pressure and osmolality
Josh’s script on Tight Junctions:
For all the love we give to ion channels and other transcellular processes, it’s paracellular transport of fluid through tight junctions does the most work of reabsorbing salt and water in the proximal tubule.
Tight junctions are ways that two opposing cells can get close together and stay there so that they create a barrier. This creates an inside the cells “lumen” and an outside the cells “interstitium”. It also creates two domains of the cell membrane—an “apical” lumen-facing side and a “basolateral” interstitium facing side. These become important because certain transporters are only localized to one of these sides. I think we’ll spend a lot of time talking about these side-specific ion channels and transporters in later installments.
What I want to spend a few minutes on is the tight junction itself. I hadn’t realized how mysterious these things were. One review I saw called them “the most enigmatic of all adhesion complexes.” There’s still a lot we don’t know about how they work. What we do know is that each of the two cells involved in forming a tight junction expresses a transmembrane protein called a Claudin. The extracellular domains of claudins fit together like the teeth of a zipper. A tight junction between two cells is made up of multiple Claudin zippers; the more zippers present, the tighter the junction.
While these junctions are called “tight” they actually still let some things through. They do this through two mechanisms: called “pores” and “leaks"
I want to talk about pores first. Just like individual particles of sand can get in between the teeth of a coarse toothed zipper, really small molecules, like single ions, can get through the pores formed by the Claudin teeth of a tight junction. Some claudin-claudin junctions allow for more pore-permeability, and some allow for less. Some claudins seem to let only cations through, while others only let anions through. This ends up having clinical implications. Genetic deficiency of the magnesium-permeable Claudin, Claudin 16, results in tight junctions that don’t allow for normal magnesium reabsorption; this means that more magnesium remains in the tubule, and magnesium is wasted into the urine.
The other mechanism by which tight junctions allow solutes to pass along the paracellular pathway is called “leakiness”. Leakiness is generated when tight junctions unzip, allowing cargos to move away from the lumen, and then re-zip, stopping the solutes from going back into the lumen. Like the opening and closing of locks in a canal, this process of opening and closing the individual Claudin zippers allows larger molecules to transit from the lumen to the interstitium. We still don’t understand how Claudin zippers decide to unzip and re-zip, but the number of zippers and the frequency of zipping probably play major roles in the transport of large solutes along the paracellular pathway.
Different epithelial tight junctions have different levels of tightness. I was surprised to find out that really tight epithelia, like the ones in the bladder (where no fluid reabsorption occurs) have an electrical resistance that’s 50,000 times higher than the very leaky epithelia of the proximal tubule. The not-so-tight junctions of the proximal tubule are primed to reabsorb salt and water, and the tighter junctions of the downstream segments are critical to creating the barriers that allow for selective reabsorption of osmoles.
However, it’s not clear that tight junctions are needed to create an apical and basolateral polarity—newer evidence shows that single cells in culture can still polarize (and because they don’t have neighbors, they don’t have tight junctions).