Chapter Ten: Acid-Base Physiology
References
We did not mention many references in our discussion today but our listeners may enjoy some highlights below.
Effects of pH on Potassium: New Explanations for Old Observations - PMC although the focus of this article is on potassium, this elegant review by Aronson and Giebisch reviews intracellular shifts as it relates to pH and K+.
Josh swooned for Figure 10-1 which shows the relationship between [H+] and pH. You can find this figure in the original reference from Halperin ML and others, Figure 1 here. Factors That Control the Effect of pH on Glycolysis in Leukocytes
Here’s Leticia Rolon’s favorite Henderson-Hasselbalch calculator website: Henderson-Hasselbalch Calculator | Buffer Solutions [hint! for this site, use the bicarbonate (or “total CO2”) for A- and PCO2 for the HA] There’s also a cooking tab for converting units!
Fundamentals of Arterial Blood Gas Interpretation - PMC this review published posthumously from the late but beloved Jerry Yee and his group at Henry Ford Hospital, explores the details and underpinnings of our understandings of arterial blood gas interpretation (and this also addresses how our colleagues in clinical chemistry measure total CO2 - which JC referenced- but JC said “machine” and our colleagues prefer the word “instrument.”)
Amy went deep on bicarbonate in respiratory acidosis. Here are her refs:
Bicarbonate Therapy in Severe Metabolic Acidosis | American Society of Nephrology this review article from Sabatini and Kurtzman addresses the issues regarding bicarbonate therapy including theoretical intracellular acidosis.
Bicarbonate in DKA? Don’t do it: Bicarbonate in diabetic ketoacidosis - a systematic review
Here’s a review from Bushinsky and Krieger on the effect acidosis on bone
https://www.sciencedirect.com/science/article/abs/pii/S0085253822002174
Here is the primary resource that Anna used in here investigation of meat replacements Nutritional Composition of Novel Plant-Based Meat Alternatives and Traditional Animal-Based Meats
We enjoyed this paper that Dr. Rose references from the Journal of Clinical Investigation 1955 in which investigators infused HCl into nephrectomized dogs and observed changes in extracellular ions. https://www.jci.org/articles/view/103073/pd
We wondered about the amino acids/protein in some available meat alternatives they are explored in this article in the journal Amino Acids: Protein content and amino acid composition of commercially available plant-based protein isolates - PMC and you may enjoy this exploration of the nutritional value of these foods: Full article: Examination of the nutritional composition of alternative beef burgers available in the United States
Outline
Chapter 10: Acid-Base Physiology
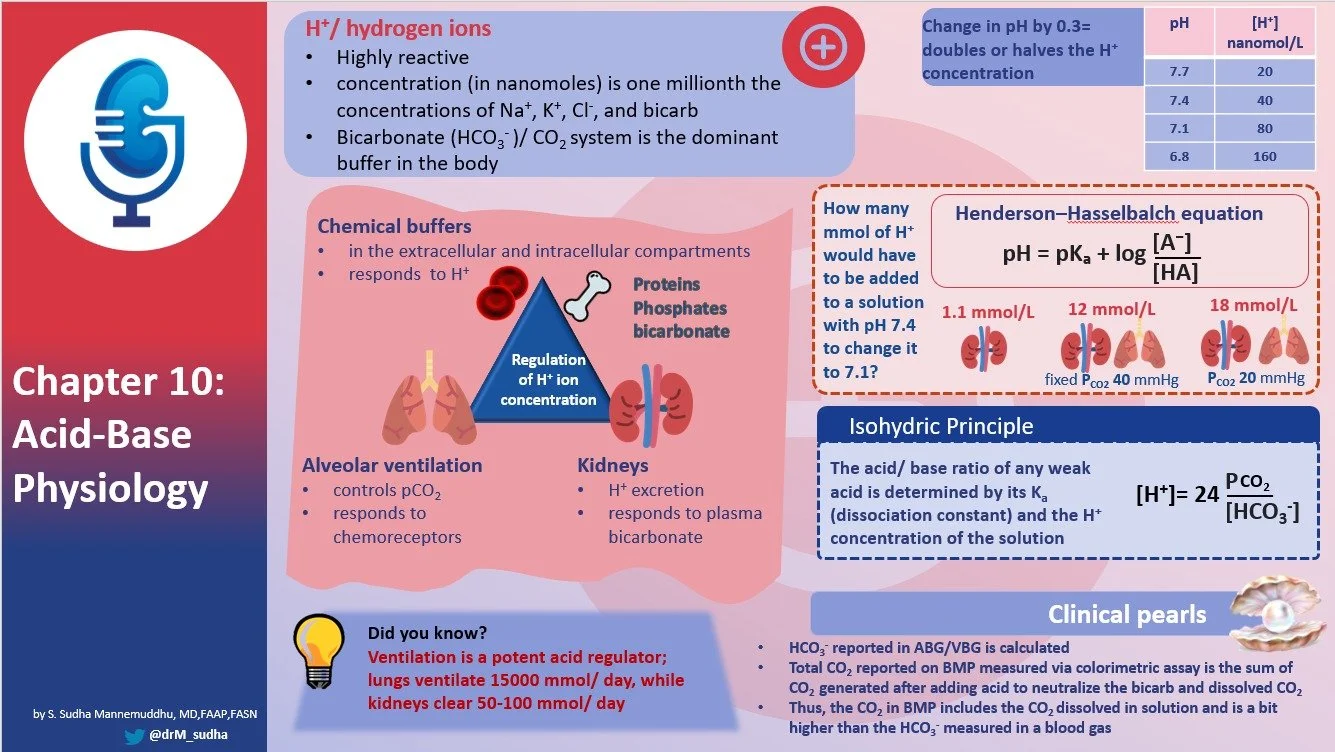
- H concentration regulated tightly
- Normal H+ is 40 nm/L
- This one millionth the concentration of Na and K
- It needs to be this dilute because H+ fucks shit up
- Especially proteins
- Cool foot note H+ actually exists as H3O+
- Under normal conditions the H+ concentration varies little from normal due to three steps
- Chemical buffering by extracellular and intracellular bufffers
- Control of partial pressure of CO2 by alterations of alveolar ventilation
- Control of plasma bicarbonate by changes in renal H+ excretion
- Acid and bases
- Use definition by Bronsted
- Acid can donate protons
- Base can accept protons
- There are two classes of acids**
- Carbonic acid H2CO3
- Each day 15000 mmol of CO2 are generated
- CO2 not acid but combines with water to form carbonic acid H2CO3
- CO2 cleared by the lungs
- Noncarbonic acid
- Formed from metabolism of protein. Sulfur containing AA generate H2SO4. Only 50-100 mEq of acid produced from these sources.
- Cleared by the kidneys
- Law of Mass Action
- Velocity of reaction proportional to the product of the concentrations of the reactants
- Goes through mass action formula for water
- Concludes that water has H of 155 nanoM/L, more than the 40 in plasma
- Says you can do the same mass experiment for every acid in the body
- Can do it also for bases but he is not going to.
- Acids and Bases can be strong or weak
- Strong acids completely dissociate
- Weak acids not so much
- H2PO4 is only 80% dissociated
- Weak acids are the principle buffers in the body
- Then he goes through how H is measured in the blood and it becomes clear why pH is a logical way to measure.
- Then there is a lot of math
- HH equation
- Derives it
- Then uses it to look at phos. Very interesting application
- Buffers
- Goes tot he phosphate well again. Amazing math describing how powerful buffers can be
- Big picture the closer the pKa is to the starting pH the better buffer, i.e. it can absorb lots of OH or H without appreciably changing pH
- HCO3 CO2 system
- H2CO3 to H + HCO3 has a PKA of 2.72 but then lots of Math and the bicarb buffer system has a pKa of 6.1
- But the real power of the bicarb buffer is that it is not a sealed system. The ability to ventilate and keep CO2 constant increases the buffering efficiency by 11 fold and the ability to lower the CO2 below normal increases 18 fold.
- Isohydric principle
- There is only one hydrogen ion concentration and since that is a critical part of the buffer equation, all buffer eq are linked and you can understand all of them by understanding one of them. So we just can look at bicarb and understand the totality of acid base.
- Bicarb is the most important buffer because
- High concentration in plasma
- Ability for CO2 to ventilate
- Other buffers include
- Bone
- Bone is more than just inorganic reaction
- Live bone releases more calcium in response to an acid load than dead bone
- More effect with metabolic acidosis than respiratory acidosis
- Hgb
- Phosphate
- Protein
RIP Mito 😢